Posted January 23, 2026 in Fertility Blog & Information
16 minute read
Key Takeaways
- Adenomyosis commonly reduces IVF success by disrupting uterine architecture and implantation. Accurate diagnosis with transvaginal ultrasound or MRI should guide treatment planning.
- Pretreatment can help. Think hormonal suppression or selective surgery depending on disease severity and fertility goals, and follow ovarian reserve closely.
- Frozen embryo transfer is often preferable to fresh transfer because it permits endometrial recovery and may increase implantation and live birth rates in affected patients.
- Miscarriage and obstetric complication risks are higher with adenomyosis, particularly with deeper lesions or increased uterine volume. This makes increased pregnancy surveillance imperative.
- Embryo quality is typically unaffected by adenomyosis, so embryo selection is still important. Uterine receptivity and timing are the key limiting factors.
- Handle the emotional cost by establishing grounded expectations, referencing your progress checklist, and developing a support network that includes therapy and peer groups.
Adenomyosis IVF outcomes cover adenomyosis’ impact on fertility treatment success and gestation. Studies report lower implantation and live birth rates with diffuse adenomyosis and higher risks of miscarriage and preterm birth.
Focal adenomyosis appears to have less obvious influence. GnRH suppression or surgery may improve outcomes, but data is inconsistent.
Below, we review the existing data, practical management steps, and patient counseling points.
Adenomyosis Impact
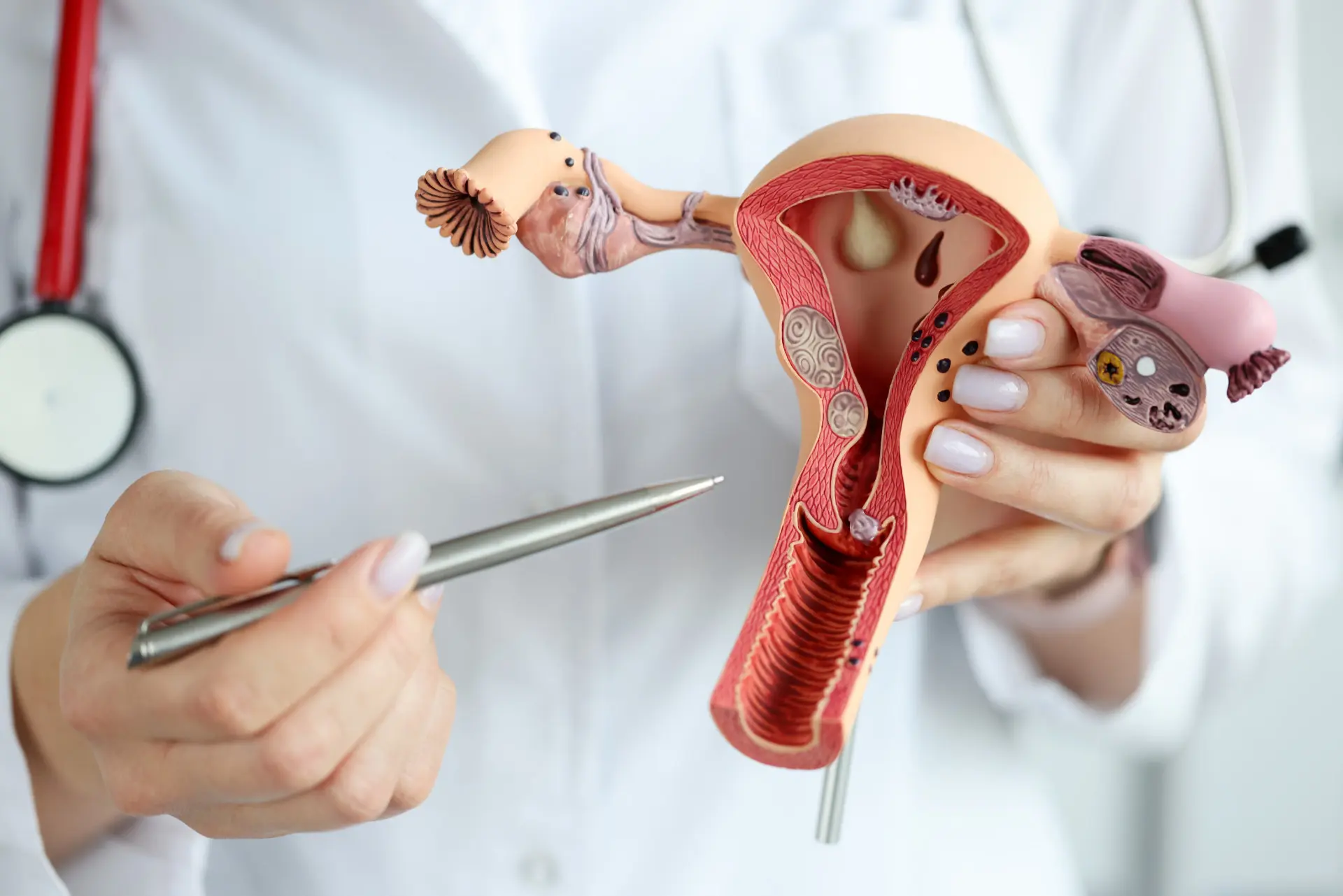
Adenomyosis alters the uterine microenvironment by enabling endometrial glands and stroma to invade the myometrium, thereby affecting tissue stiffness, vascular distribution and local inflammatory cues. This aberrant environment diminishes fertility and may exacerbate IVF success through mechanical distortion and biochemical modifications at the endometrial–myometrial interface.
Adenomyosis occurs in 7% to 27% of infertile women and up to 32% of IVF populations, so this impact is clinically applicable to many patients.
1. Implantation Failure
Abnormal uterine contractility and a disturbed endometrium make it difficult for an embryo to implant and thrive. Uterine peristalsis can be uncoordinated, resulting in poor apposition of the embryo and local inflammatory cytokines interfere with trophoblast invasion.
Both adenomyotic tissue invading the myometrium and an enlarged uterine volume decrease implantation rates. There are reported implantation rate differentials of 25.6% with adenomyosis versus 28.6% with controls.
MUSA features, such as junctional zone thickening, myometrial cysts, and asymmetric wall thickening, correlate with RIF and can help direct clinical decisions. Abnormal implantation is a key cause of infertility in these patients and detecting sonographic markers pre-transfer can alter management such as selecting freeze-all cycles or extended suppression.
2. Ovarian Response
Ovarian response to stimulation in women with adenomyosis is frequently comparable to controls but could be a bit lower depending on age and disease severity. The number of oocytes collected and available embryos has been described as similar between adenomyosis patients and controls, suggesting egg quality is not consistently impacted.
Different stimulation protocols such as antagonist versus long agonist regimens can also influence outcomes, with some clinics noting modest benefits with long protocols and pretreatment suppression. Others observe minimal differences.
Ovarian reserve markers like AMH and antral follicle count should be monitored and tracked closely to customize stimulation. A straightforward table of oocyte yield, fertilization rate, and cleavage rate is easy to understand and can highlight the actual expected outcome in practice when counseling patients.
3. Miscarriage Risk
Adenomyosis raises miscarriage risk. Pooled data show miscarriage rates around 29.1% versus 17.2% without the disease. Uterine wall defects, altered endometrial receptivity, and impaired decidualization all contribute to this higher loss even with good embryo quality.
Risk persists after fresh and frozen transfers. Severe adenomyosis and greater uterine volumes additionally increase the risk of pregnancy loss. Therefore, risk stratification and close monitoring are critical.
4. Pregnancy Complications
Adenomyosis elevates obstetric risks such as preterm delivery and greater incidence of antepartum hemorrhage and placental abnormalities. Higher rates of extreme preterm delivery occur, and in certain series, there are uncommon occurrences such as uterine rupture.
Pregnancy follow-up should be intensified, with monitoring of placental position and fetal growth, and delivery planning personalized to each case.
5. Embryo Quality
Adenomyosis itself does not directly reduce embryo quality. The majority of evidence demonstrates similar oocyte counts and embryo availability between groups.
Embryo consideration, including blastocyst grade, shape, and rate, continues to be key. Cumulative pregnancy rates are not only reliant on embryo quality but on uterine receptivity. Maximizing both is crucial for increasing live birth rates.
Diagnostic Clarity
To begin with, it is important to accurately diagnose adenomyosis prior to commencing IVF. Imaging-driven confirmation alters counseling, pretreatment decisions, and cycle timing. Misdiagnosis results in misguided interventions or lost chances to do better.
Imaging Techniques
Transvaginal ultrasound, magnetic resonance imaging (MRI), and 3D sonography are the primary tools for detecting adenomyosis. Each shows hallmarks such as uterine enlargement, focal myometrial lesions, and subendometrial myometrial striations. Identification of these features guides further management.
Ultrasound is widely available and useful as a first-line test. Transvaginal scans can show myometrial cysts, heterogeneous myometrium, and asymmetry between anterior and posterior walls. A screening ultrasound can pick up many features linked to poorer IVF results, but using ultrasound alone risks false positives and negatives because fibroids or scarring can mimic adenomyosis.
MRI provides enhanced soft tissue contrast and better characterizes junctional zone thickening and lesion invasive depth. It’s more dependable when ultrasounds are ambiguous or when accurate mapping is required for surgery.
3D sonography provides additional spatial detail and can enhance detection of subendometrial striations that foreshadow negative IVF-ET outcomes even with mild thickening of two to two point four nine centimeters.
| Modality | Strengths | Limitations | Reported accuracy |
|---|---|---|---|
| Transvaginal US | Readily available, good screening | Operator-dependent, mimicry by fibroids | Variable; risk of false results |
| 3D Sonography | Better spatial detail, junctional zone views | Requires equipment/expertise | Improved over 2D US in some studies |
| MRI | High soft-tissue contrast, precise mapping | Costly, less accessible | Highest diagnostic accuracy in severe cases |
Repeat imaging or complementary modalities enhance diagnostic clarity. The predictive model performed well with an area under the curve of 0.915 in the modeling cohort and 0.940 in validation, reinforcing the promise of multimodal approaches for consistent diagnosis.
Severity Grading
Severity grading should take into account lesion depth, uterine volume, and extent of myometrial involvement as they correlate directly with reproductive outcomes. Deeper lesions and larger uterine volumes are strongly correlated with lower implantation rates and higher miscarriage risk.
Reported adenomyosis miscarriage rates range from approximately 29.1 percent to 82.4 percent in affected cohorts. By applying standardized definitions, we minimize this variation. Different diagnostic criteria account for the variation in prevalence, which ranges from 8% to 27% in general populations and is 23% or more in some studies.
In IVF populations, prevalence can be higher. One study identified 32% among women undergoing IVF. Severity grading informs pretreatment choices such as hormonal suppression, surgical options, or modified embryo transfer timing.
Consistent use of graded assessments across centers allows better outcome comparisons and tailored IVF cycle planning that may improve chances of live birth.
Pretreatment Protocols
To treat pretreatment, we try to decrease adenomyotic bulk, decrease inflammation, and optimize the uterine lining prior to ovarian stimulation and transfer. The decision of pretreatment is based on adenomyosis subtype, size, symptom load, history of prior surgery or attempted resection, and the patient’s future fertility desire.
Hormonal Suppression
Hormonal suppression with GnRh agonists or progestins can shrink adenomyotic tissue and restore uterine receptivity by minimizing estrogen-driven growth and local inflammation.
| Regimen | Typical duration | Mechanism | Reported impact on IVF outcomes |
|---|---|---|---|
| Ultra-long GnRHa protocol | ≥3 months before stimulation | Profound hypoestrogenism, tissue shrinkage | Some studies show improved outcomes; others report reduced clinical pregnancy (CPR) and live birth rates (LBR) in adenomyosis cases |
| Long-acting GnRHa | 1–6 months | Sustained suppression | Used widely; shown to be protective for LBR in some analyses when used without surgery |
| Continuous progestin (oral or LNG-IUD) | Variable, often months | Endometrial decidualization, reduced inflammation | LNG-IUD may lower uterine inflammation and improve implantation environment |
Contrast these regimens in terms of length, extent of suppression, side effects and monitoring requirements. For instance, ultra-long GnRHa administered for a minimum of three months seeks to optimize shrinkage, with 28 to 35-day repetitions occasionally extended until CA-125 levels normalize.
Some cohorts saw GnRHa alone protective for LBR, but others observed LBR lower than no pretreatment. From a practical perspective, progestin LNG-IUDs are appealing to symptomatic patients who prefer local control and the possibility of implantation improvement without systemic hypoestrogenism.
Surgical Options
Surgical options consist of adenomyomectomy and uterine reconstruction in select cases of localized disease or when anatomy is significantly distorted. Surgery restores normal uterine contour and cavity, potentially increasing implantation rates in subsequent IVF cycles.
Risks such as intrauterine scarring, thinning of the uterine wall, and potential cesarean delivery are weighed against the fertility benefits. Suggest surgery for focal adenomyosis, failure of medical therapy or when big lesions compress the cavity.
Diffuse disease typically does best with medical pretreatment or combination methods. Data imply conservative surgery plus GnRHa has higher LBR than GnRHa alone in some series.
Non-Hormonal Aids
Non-hormonal aids like acupuncture, anti-inflammatories like NSAIDs strategically timed, and dietary changes target reducing systemic inflammation and enhancing overall reproductive health. The data is sparse, but encouraging when these are applied as adjuncts.
Studies find modest symptom relief and some IVF-related benefits when accompanied by medical pretreatment protocols. Incorporate these adjunctives with hormonal or surgical pretreatment — not as monotherapy.
Watch for interactions. For example, NSAIDs around ovulation can impact follicular rupture, so timing is important. Follow results and side effects seriously and customize adjuncts to patient inclination and culture.
Transfer Timing
Transfer timing influences IVF implantation and live birth chance for women with adenomyosis. There is a trade-off when deciding the timing of embryo placement between embryo stage, uterine recovery, and the mother’s hormonal milieu. Below are targeted trade-offs between fresh and frozen approaches and how timing can minimize adenomyosis damage.
Fresh Transfer
Fresh embryo transfer might be worse in adenomyosis because ovarian stimulation frequently leaves the uterus inflamed and more contractile. Hyper-uterine contractility can shove out embryos or interrupt the implantation window. Multiple cohort studies observe reduced implantation and clinical pregnancy rates in adenomyosis patients with fresh transfers.
In one comparative study, implantation rates were significantly different, with 25.6% in one group and 28.6% in the other group, with a P value of 0.027. This difference likely relates to the acute effects of stimulation. Ovarian hyperstimulation increases estradiol and inflammatory mediators. These changes can still be around during a new transfer and dull responsiveness.
Embryo age matters too: most transfers are done on day 5 (60.8%), with fewer on day 3 (37.7%) and rare day 4 (1.5%). Although there are data in support of day-5 blastocyst transfer for higher pregnancy rates, this advantage can be negated in fresh cycles when the uterine lining is less than optimal. Because of these issues, clinicians look for other options.
For instance, switching to all-freeze cycles when the endometrium appears thin or inflamed while stimulating can prevent implanting embryos into a toxic uterus. That strategy aims to isolate embryo quality from temporary stimulation-induced uterine dysfunction.
Frozen Transfer
Frozen embryo transfer (FET) allows for endometrial recovery time and can optimize uterine receptivity in adenomyosis. Postponing transfer gives the inflammation time to calm down and the hormones a chance to level out. Preparatory regimens—natural cycles, hormone replacement, or GnRH suppression—can be customized to the woman.
Studies show higher cumulative pregnancy rates and live birth with frozen transfers in this population, and vitrification has increased embryo survival post-thaw. Blastocyst transfers (day 5) are common in FET protocols because they can align with the natural implantation window and have been shown to have better implantation rates than day-3 transfers at many centers.
Convenient scheduling and the ability to optimize endometrial receptivity tests are among other practical advantages. Drawbacks include that FET requires extra time, cost, and sometimes additional medications. Some women respond poorly to hormonal replacement or have unpredictable natural cycles.
All in all, for a lot of women with adenomyosis, frozen transfer is best to enhance outcomes and minimize risks at the uterine level.
The Emotional Toll
Adenomyosis introduces a medical component to infertility that has distinct emotional undertones. Qualitative differences in outcomes, such as a lower clinical pregnancy rate of 47.06% compared to 64.42%, a higher spontaneous abortion rate of 33.33% compared to 13.43%, and a lower live birth rate of 31.37% compared to 54.81%, color how patients perceive IVF.
These numbers frequently translate to recurrent heartbreak, mourning over miscarriages, and persistent doubt around what comes next.
Managing Expectations
Patients require a pragmatic context for what IVF can do amid adenomyosis. Clinicians should discuss that there is a success rate based on severity of disease, uterine anatomy, and treatment plan, meaning whether surgery or medical treatment is done before IVF.
Instead, set goals based on process milestones, such as finishing a stimulation cycle, undergoing an embryo transfer, and receiving a biochemical pregnancy, as opposed to just live birth.
A realistic to-do list keeps objectives tangible and minimizes obsessive thought. Items can include: dates of scans and blood tests, embryo quality grades, medication schedules, emotional check-ins, and next-step contingencies if cycles fail.
Go over the list with the care team at every appointment and update targets as new data arises. Tracking with short-term metrics builds small wins. For example, celebrate completing a medication protocol or improving sleep and stress markers, even if pregnancy is not achieved.
This mindset minimizes the all-or-nothing thinking that makes setbacks feel devastating.
Building Support
Being part of a support group provides access to those who know the mixture of medical and emotional stress. Online or local groups around adenomyosis or IVF let you swap tips from taming side effects to navigating clinic admin and normalize the roller-coaster of hope and grief.
By bringing in partners and trusted friends, you’re constructing a safety net for hard days. Simple actions matter: a partner attending an appointment, a friend driving after a procedure, or family members providing meals.
These behaviors reduce daily stress and can enhance treatment compliance. Professional counseling, while often underutilized, can be instrumental. Reproductive-experienced therapists assist with coping strategies, post-miscarriage grief, and anxiety-reducing cognitive tools.
Clinics that provide or refer to mental health services improve patient-reported outcomes. Discussing resilience tips among the adenomyosis community eases its emotional impact. Tales of surviving flamed cycles, when to take a break from treatment or how to request a second opinion offer templates patients can customize.
Checklists co-shared by peers, coping routines, and clinician referrals all become immediately actionable resources patients can use.
Future Outlook
Diagnostic and personalized IVF care advances will define adenomyosis women’s outcomes in the future. As better imaging and more people seek care, prevalence estimates will only increase. It now ranges widely from roughly 5 to 70%, while it remains difficult to detect and is underdiagnosed. Better access to state of the art 2D and 3D TVS and optimized MRI protocols will reduce that range.
These tools already demonstrate high sensitivity and specificity compared to MRI and histology, and ongoing refinement should enable earlier, clearer determination of disease extent and subtype, which is important for fertility care planning. Better imaging fuels more precise IVF protocols. Better maps of adenomyotic lesions will allow clinicians to select stimulation, pretreatment, and transfer timing more precisely.
New evidence indicates that adenomyosis could have a minimal impact on implantation rates but is associated with increased early miscarriage rates. For instance, one such prospective cohort discovered similar implantation rates but increased early miscarriages. Clinically, that indicates protocols might trend toward more aggressive suppression prior to transfer or toward frozen embryo transfer approaches that allow the uterus to heal.
Today’s rates, clinical pregnancy approximately 30% and live birth close to 25% for a Day 5 fresh transfer, provide a benchmark against which to measure new strategies. Pretreatment and procedural decisions will presumably be further honed by trials juxtaposing hormonal suppression, conservative surgery, and interventional radiology.
Conservative surgical repair has held promise, as an analysis reported pooled pregnancy and live birth rates of approximately 53.4 percent and 35.2 percent, respectively. Uterine artery embolization (UAE) yielded significant symptom relief in approximately 83.1 percent of patients in a meta-analysis, with technique refinements potentially shifting its fertility role.
Drug candidates being investigated, including ulipristal acetate, mifepristone, and levonorgestrel intrauterine systems, could provide noninvasive methods of suppressing lesion activity and optimizing the uterine environment before IVF. New targeted treatments are a very real possibility.
Research on agents that act on local inflammation, angiogenesis, or progesterone resistance might decrease adenomyotic activity without definitive surgery. Local delivery, new selective receptor modulators, and minimally invasive ablation methods are all being investigated. Where these are available, they can be paired with optimized embryo selection and endometrial preparation to minimize miscarriage and increase live births.
Continued studies will shed light on how to optimize combinations of imaging, medical management, surgery and IVF timing to best impact outcomes. As data accrue, clinicians will provide more personalized plans considering symptom control, fertility desire and risks.
Conclusion
Adenomyosis may slash IVF outcomes. Specific measures can increase the chances. Proper scans and uniform criteria assist in detecting it early. Hormone pretreatment and controlled ovarian stimulation are beneficial in many of the studies. Frozen transfer after endometrial recovery usually does better than fresh. Emotional strain runs deep, and a rock-solid support system and honest communication with a care team relieve tension and maintain optimism. Active trials will hone which medications and timing work best. In the meantime, choose a clinic that employs transparent imaging, provides customized pretreatment, and monitors results. Walk through risks, expenses, and a plan that suits your body and life. Contact your care team to plan the road ahead.
Frequently Asked Questions
How does adenomyosis affect IVF success rates?
Adenomyosis can reduce implantation and live birth rates. It depends on severity and age. Pretreatment and customized protocols can enhance results for the majority.
Can adenomyosis be accurately diagnosed before IVF?
Yes. Diagnosis uses transvaginal ultrasound and MRI. Expert imaging and gynecologists improve accuracy and treatment planning.
Do any pretreatments improve IVF outcomes with adenomyosis?
Yes. GnRH agonist therapy for 2 to 6 months and targeted surgery in select cases can improve implantation and live birth rates. Talk about the pros and cons with your specialist.
Should embryo transfer be fresh or frozen for women with adenomyosis?
Most studies support frozen embryo transfer following medical pretreatment. This gives the endometrium a chance to recover and could improve the odds of pregnancy. Personal factors direct the ultimate choice.
How does adenomyosis affect miscarriage risk?
Adenomyosis is associated with an increased rate of early pregnancy loss. Pretreatment and personalized IVF protocols can mitigate this risk but do not negate it.
What emotional challenges should patients expect during IVF with adenomyosis?
There is a lot of anxiety, grief, and uncertainty for patients. Open communication, counseling, and support groups alleviate stress and enhance coping.
Are there promising future treatments for adenomyosis-related infertility?
Yes. Studies investigate better imaging, minimally invasive surgery, targeted drugs, and fertility-sparing strategies. Current trials could open doors in the coming years.
